Tick-Borne Disease: Tremendously Tricky in Horses
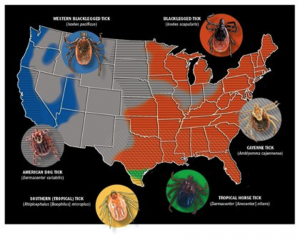 Maps courtesy of Centers for Disease Control and Prevention; Tick photos courtesy of Mat Pound/USDA/Bugwood.org and Wikimedia Commons
Learn the latest on diseases horses can get from ticks and why they continue to frustrate veterinarians and researchers
If the sight of a tick makes your skin crawl—even if it’s not crawling on your skin— you’re not alone. That feeling is founded on more than a natural aversion to arachnids; diseases transmitted by ticks can pose a real health threat. With Centers for Disease Control and Prevention (CDC) maps outlining tick ranges throughout the majority of the United States, it’s important we brush up on our understanding of tick-borne diseases. In this article we’ll take a look at the three that pose the biggest risk to horses: Lyme disease, anaplasmosis, and piroplasmosis.
Lyme Disease
Horse owners living in areas of the country heavily infested with Ixodes scapularis, commonly known as blacklegged ticks (also referred to as deer ticks or bear ticks), know these parasites are more than a nuisance. In these regions contracting Lyme disease from infected ticks is entirely possible for horses and humans alike.
Lyme disease is a very difficult disease to prevent, diagnose, and treat in horses, says Linda Mittel, MSPH, DVM, senior extension associate at Cornell University’s Animal Health Diagnostic Center, in Ithaca, New York. Horses contract Lyme disease when the spirochete (a type of bacterium) Borrelia burgdorferi is transmitted through the bite of an infected tick.
“Signs of Lyme disease might not appear until 6 weeks after exposure”
Diagnosis Here’s why diagnosing Lyme disease is tricky: Not all infected horses exhibit clinical signs, and the signs themselves are often confounding, meaning they can point to any number of other diseases. Even if you know an infected tick bit your horse (You can actually test it!), signs of Lyme disease might not appear for up to six weeks after exposure. In short, nothing is straightforward with this disease. An infected tick might or might not transmit the bacteria to its host, and an infected animal might or might not exhibit signs of disease.
So while Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye). While in private practice in the Northeast, Mittel often saw non-neurologic clinical signs of Lyme disease that included unexplained weight loss, changes in attitude and behavior, uveitis, and hypersensitivity. Some signs might arise from the bacterial infection itself, while others (e.g., uveitis) result from the body’s immune response to it. Lyme neuroborreliosis (NB), a rare condition, occurs when B. burgdorferi infects the horse’s nervous system.
Maps courtesy of Centers for Disease Control and Prevention; Tick photos courtesy of Mat Pound/USDA/Bugwood.org and Wikimedia Commons
Learn the latest on diseases horses can get from ticks and why they continue to frustrate veterinarians and researchers
If the sight of a tick makes your skin crawl—even if it’s not crawling on your skin— you’re not alone. That feeling is founded on more than a natural aversion to arachnids; diseases transmitted by ticks can pose a real health threat. With Centers for Disease Control and Prevention (CDC) maps outlining tick ranges throughout the majority of the United States, it’s important we brush up on our understanding of tick-borne diseases. In this article we’ll take a look at the three that pose the biggest risk to horses: Lyme disease, anaplasmosis, and piroplasmosis.
Lyme Disease
Horse owners living in areas of the country heavily infested with Ixodes scapularis, commonly known as blacklegged ticks (also referred to as deer ticks or bear ticks), know these parasites are more than a nuisance. In these regions contracting Lyme disease from infected ticks is entirely possible for horses and humans alike.
Lyme disease is a very difficult disease to prevent, diagnose, and treat in horses, says Linda Mittel, MSPH, DVM, senior extension associate at Cornell University’s Animal Health Diagnostic Center, in Ithaca, New York. Horses contract Lyme disease when the spirochete (a type of bacterium) Borrelia burgdorferi is transmitted through the bite of an infected tick.
“Signs of Lyme disease might not appear until 6 weeks after exposure”
Diagnosis Here’s why diagnosing Lyme disease is tricky: Not all infected horses exhibit clinical signs, and the signs themselves are often confounding, meaning they can point to any number of other diseases. Even if you know an infected tick bit your horse (You can actually test it!), signs of Lyme disease might not appear for up to six weeks after exposure. In short, nothing is straightforward with this disease. An infected tick might or might not transmit the bacteria to its host, and an infected animal might or might not exhibit signs of disease.
So while Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye). While in private practice in the Northeast, Mittel often saw non-neurologic clinical signs of Lyme disease that included unexplained weight loss, changes in attitude and behavior, uveitis, and hypersensitivity. Some signs might arise from the bacterial infection itself, while others (e.g., uveitis) result from the body’s immune response to it. Lyme neuroborreliosis (NB), a rare condition, occurs when B. burgdorferi infects the horse’s nervous system.
 While Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye).
Photo: iStock
Researchers have made strides in Lyme disease diagnostic testing, but there’s still a long way to go. The newest test for B. burgdorferiantibodies (the immune system produces antibodies to fight antigens, or foreign substances, it detects) in the blood is the equine Lyme Multiplex assay developed by the Animal Health Diagnostic Center. This screening identifies three antigen proteins:
While Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye).
Photo: iStock
Researchers have made strides in Lyme disease diagnostic testing, but there’s still a long way to go. The newest test for B. burgdorferiantibodies (the immune system produces antibodies to fight antigens, or foreign substances, it detects) in the blood is the equine Lyme Multiplex assay developed by the Animal Health Diagnostic Center. This screening identifies three antigen proteins:
- “Outer surface protein A” antibodies, which were previously thought to be found in vaccinated horses, but now the research is undecided;
- “Outer surface protein C” antibodies in recently infected horses, which are present within three to five weeks of infection, declining by seven to 11 weeks and no longer present by five months; and
- “Outer surface protein F” antibodies in horses with chronic infection, identifiable from two to three months on.
These results leave room for interpretation. “What is considered positive is a little hard to specifically define,” notes Mittel. “If one of these areas, particularly the outer-surface protein F, is positive, it doesn’t mean it’s causing disease in the horse; it simply means the animal has been exposed. If the animal is showing clinical signs associated with Lyme disease, in many cases the outer-surface protein F will be elevated, but not always. It’s a very difficult disease to diagnose, because we are, at this point in time, diagnosing with an ‘all antibody test’—not testing for the organism (itself) in the body, but for the animal’s response to the organism. Antibodies mean the horse has been exposed to the agent. It’s possible the animal will be ill, but a positive doesn’t necessarily mean the horse needs to be treated.”
The good news is that the turnaround on the Multiplex test is less than a day. “This gives you an idea if the animal has been exposed,” says Mittel, allowing the veterinarian to begin treatment immediately if the horse has compatible clinical signs.
While the Lyme Multiplex is the exciting new kid on the block, another test provides the most definitive analysis. “The Western blot test is the most comprehensive test for antibodies to B. burgdorferi and enables the diagnostician to determine the presence of antibodies produced to all of the major protein components of the organism, rather than the three proteins targeted by the Multiplex test,” says Sandra Bushmich, DVM, MS, section head at the University of Connecticut’s Veterinary Medical Diagnostic Laboratory, in Storrs. “This can prove especially advantageous when following the course of an infection over time or determining response to antibiotic treatment. Because of its increased complexity, the Western blot test takes a few days to run and requires a laboratory with particular expertise in equine Lyme Western blot testing.”
“The Western blot test is the most comprehensive test for antibodies to B. burgdorferi.”
- SANDRA BUSHMICH
In addition to Lyme Multiplex and Western blot diagnostics, other available initial screening tests include the canine IDEXX SNAP; the enzyme-linked immunosorbent assay (ELISA); and the indirect fluorescent antibody tests (IFAT), all of which can detect B. burgdorferi antibodies, indicating exposure to Lyme disease.
If you find a tick on your horse and save it for evaluation, your veterinarian can submit it to a reference lab for a polymerase chain reaction (PCR) test. This checks the tick for B. burgdorferi DNA. A positive reading indicates it is a vector (carrier) of Lyme disease. This means the horse might have been exposed to the bacteria, but he won’t necessarily develop the condition, explains Mittel. Labs can even use PCR tests to test for the organism within the horse, including within cerebrospinal fluid, joint fluid, and joint tissue, although Mittel notes this procedure is relatively uncommon. Beyond all these testing options, she emphasizes that the veterinarian’s evaluation of the animal and ruling out conditions that produce similar signs is of primary importance.
A licensed vaccine providing protection against B. burgdorferi would be a welcome addition to horse vaccination protocols, but currently none is available. Some veterinarians vaccinate horses using a canine product off-label, but this procedure is not without concerns, and its efficacy is questionable. Mittel explains that if a vaccinated horse does develop Lyme disease symptoms, his vaccination status could confound the equine Lyme Multiplex data.
Treatment For treatment Mittel says veterinarians most commonly prescribe oral doxycycline and minocycline, both antibiotics. Veterinarians can also administer intravenous oxytetracycline, which, Mittel explains, like all tetracycline antibiotics, has an additional anti-inflammatory response. “If the horse has another condition, like arthritis, which is causing inflammation, and it’s not clear if lameness is due to Lyme disease or another condition, the horse may appear improved after treatment—you may have a false impression that the horse had Lyme disease, when it’s improving to the side effect of drug,” says Mittel. Or, if the horse stays sound after finishing treatment, Lyme disease might have been making him lame.
In tick-infested areas, horse owners should check horses often that have been on pasture or in brushy and wooded areas. Remove ticks immediately. According to the CDC website, transmission of the B. burgdorferi bacterium occurs 36-48 hours after the tick has begun feeding.
Anaplasmosis
Equine granulocytic anaplasmosis, another tick-borne bacterial disease, caused by Anaplasma phagocytophilum (previously known as Ehrlichia equi, with the condition called equine granulocytic ehrlichiosis), is spread by the bite of infected I. scapularisand I. pacificus (Western blacklegged) ticks and infects the host’s white blood cells.
Clinical signs, apparent within one to three weeks of infection, might include high fever, suppressed appetite, edema (fluid swelling), depression, petechial (pinpoint) hemorrhage, and inability to walk normally. While anaplasmosis causes some signs similar to Potomac horse fever (PHF), cases tend to occur from late fall through winter and into early spring, whereas PHF occurs in late summer and early fall.
Diagnosis Veterinarians can diagnose anaplasmosis by manually evaluating a blood smear with an in-house complete blood count panel. The organism is detectable in white blood cells, explains Mittel, who notes that practitioners might also employ PCR and/or IFAT.
Bushmich describes new developments in anaplasmosis diagnosis: “Recent work in our lab has shown a PCR test on a buffy coat sample (the white blood cell layer of a centrifuged whole blood sample) is more sensitive than a PCR on whole blood or direct microscopic exam for morulae (visible inclusion bodies in the white blood cells). … The buffy coat sample PCR is a sensitive test (it produces very few false negatives); this is a really good test for an acutely ill horse (to differentiate it from from other clinically similar diseases). If the veterinarian is considering anaplasmosis, this test is fast—you can usually get it within a day or two, and it’s very specific,” says Bushmich, meaning it produces very few false positives. “Serological (antibody) tests are better for determining previous exposure or subacute (between acute and chronic), long-term cases.”
Treatment “In general, this is a self-limiting disease,” meaning it will resolve on its own, says Mittel, “but it can be critical to some horses. Anaplasma responds well to treatment. The drugs of choice are doxycycline or oxytetracycline, both of which are renal (kidney) toxic, so care must be taken during treatment, as the animal may have become dehydrated over the course of the illness.” As with Lyme disease, there is currently no equine vaccine available.
Piroplasmosis
Transmission of equine piroplasmosis (EP, also called babesiosis), a blood-borne disease caused by Babesia caballi and/or Theileria equi parasite infection, occurs when a tick feeds on an infected horse and then on an uninfected horse, or when contaminated equipment is shared among horses, such as used dental and tattoo equipment, needles, and surgical instruments. Horses can also acquire the disease from infected blood transfusions and from an infected dam during birth.
“What’s concerning is the likely spread of (piroplasmosis) between horses, which we see happening with contaminated equipment or blood doping at nonsanctioned Quarter Horse racetracks. ”
- LINDA MITTELL
Currently, the disease is not considered endemic in the United States and has occurred only in isolated outbreaks. Because the USDA has declared EP a reportable disease, veterinarians must notify authorities before collecting diagnostic samples in suspected cases. All horses entering the United States, whether temporarily or permanently, must test EP-negative to be allowed entry.
Some exceptions to this procedure have been made in the cases of international events, such as the 2010 World Equestrian Games in Kentucky, where the USDA granted EP-positive horses waivers, allowed them entry to compete, and housed and managed them under special conditions with state/federal oversight.
Various ticks have been shown, either naturally or experimentally, to be EP vectors. Chantal Rothschild, DVM, Dipl. ACVIM, of Northwest Equine Veterinary Associates, near Seattle, Washington, says that the tropical horse tick (Dermacentor [Anocentor] nitens) and Southern cattle tick (Rhipicephalus [Boophilus] microplus) are proven -transmitters, as are the Cayenne tick (Amblyomma cajennense) and American dog tick (Dermacentor variabilis). A native of Brazil who did her initial veterinary studies there but her internship and residency in the United States, Rothschild has seen EP from various angles.
Warming Temperatures
Politics aside, data indicates an ongoing trend toward warming temperatures, which in turn impacts habitats and organisms, say scientists at the Cary Institute of Ecosystem Studies in Millbrook, New York, which has released findings from its research center.
“Host-seeking behavior by ticks is inhibited by temperatures below freezing, and tick activity slows dramatically below about 40°F,” says Richard Ostfeld, PhD, a disease ecologist there. “Warmer winter temperatures mean that ticks can be active longer, which probably increases their ability to find hosts. We have found that the larval and nymphal stages (nymphs transmit the vast majority of Lyme disease) become active a few weeks earlier in warmer than in cooler years. The long-term trend has been for ticks to emerge earlier.”
Along with increased tick activity, warmer weather means expanded ranges and greater numbers of cases. “There is evidence from several studies that, as the climate warms and growing seasons lengthen, ticks are able to invade areas that were formerly too harsh,” he notes. “This is probably why ticks (and the diseases they transmit) have been steadily expanding in both latitude and altitude. The blacklegged ticks (Ixodes scapularis) that transmit Lyme disease, babesiosis, and anaplasmosis don’t appear to have strong effects on their wild animal hosts. But, the winter tick (Dermacentor albipictus) can cause declines in health of their hosts, which are mainly moose and deer, but also sometimes horses. There is some evidence that warm winter conditions increase winter tick infestations by decreasing body condition of the hosts and possibly increasing tick survival.”
NATALIE DEFEE MENDIK
Infected horses exhibit signs one to two weeks after exposure. Signs can be mild or acute, ranging from suppressed appetite and weakness to fever, anemia, edema, labored breathing, and jaundice. Mittel says cases can run from subclinical (not showing signs) to fatal.
Surviving horses, while no longer sick, can pose a biosecurity problem. “Once infected, horses tend to become carriers, even after drug treatment to resolve the clinical signs. They may be still positive years after infection,” says Rothschild. “And here lie the USDA’s concerns: Carrier horses have no clinical signs and cannot be distinguished from normal healthy horses (without diagnostic testing); however, they still have the potential to transmit the disease via ticks or contaminated equipment.
“If a horse is known to be EP-positive, it must be euthanized, exported from the U.S., entered into lifelong quarantine, or enrolled in an ongoing research -treatment program under the oversight of the USDA,” she adds.
Diagnosis Currently the C-ELISA blood test is the gold standard for EP diagnostic testing, says Rothschild. “It picks up on horses that have low levels of -infection—ones that may test negative (on the complement fixation test, a blood test used to screen horses entering the country from the 1970s to 2005) when, in fact, they are positive.” When the disease is in a chronic state, notes Rothschild, the horse’s antibody levels might fluctuate, complicating test results. DNA testing is also an option. A USDA–approved laboratory must conduct both these tests.
“What’s concerning is the likely spread of this disease between horses, which we see happening with contaminated equipment or blood doping at nonsanctioned Quarter Horse racetracks,” remarks Mittel. “The concern is then if an infected racehorse retires and becomes part of the mainstream equine population, this could lead to the spread of disease.”
Moving Forward
There is more research to be done in the field of tick-borne diseases. “I think we will probably see more newly recognized diseases as we have more diagnostic capabilities,” says Mittel. “We may see more coinfections or even three organisms in the horse. As horses move about, veterinarians need to be aware of the prior location of the horse.”
“Be on the lookout for Borrelia miyamotoi, an emerging tick-borne disease agent spread by the same Ixodes tick species that may transmit Borrelia burgdorferi,” says Bushmich. “B. miyamotoi can cause illness with fever, headache, and fatigue, generally without rash, in humans. Although it can infect animals (there is some limited data from studies done on wild animals), there is no information on domestic animals. Our laboratory is currently investigating this infection in horses. We don’t know yet if this is a problem in horses, but we think it might be.”
For example, she explains how in areas where Lyme disease is common, some horses present clinical signs similar to those seen with Lyme disease, yet ELISA and Western blot tests can’t confirm the infection. However, the horse responds well to antibiotic treatment despite a lack of serological support for a diagnosis.
In light of these threats, both known and unknown, Bushmich’s principal advice to horse owners is to remain vigilant about tick control and use topical permethrin spot-on treatments in fall and early spring, when adult Ixodes scapularis ticks are -abundant.
 ABOUT THE AUTHOR
Natalie DeFee Mendik, MA
Freelance journalist Natalie DeFee Mendik is a multiple American Horse Publications editorial and graphics awards winner specializing in equestrian media. She holds an MA in English from Colorado State University and an International Federation of Journalists’ International press card, and is a member of the International Alliance of Equestrian Journalists. With over three decades of horse experience, Natalie’s main equine interests are dressage and vaulting. Having lived and ridden in England, Switzerland, and various parts of the United States, Natalie currently resides in Colorado with her husband and two girls.
ABOUT THE AUTHOR
Natalie DeFee Mendik, MA
Freelance journalist Natalie DeFee Mendik is a multiple American Horse Publications editorial and graphics awards winner specializing in equestrian media. She holds an MA in English from Colorado State University and an International Federation of Journalists’ International press card, and is a member of the International Alliance of Equestrian Journalists. With over three decades of horse experience, Natalie’s main equine interests are dressage and vaulting. Having lived and ridden in England, Switzerland, and various parts of the United States, Natalie currently resides in Colorado with her husband and two girls.
 Maps courtesy of Centers for Disease Control and Prevention; Tick photos courtesy of Mat Pound/USDA/Bugwood.org and Wikimedia Commons
Learn the latest on diseases horses can get from ticks and why they continue to frustrate veterinarians and researchers
If the sight of a tick makes your skin crawl—even if it’s not crawling on your skin— you’re not alone. That feeling is founded on more than a natural aversion to arachnids; diseases transmitted by ticks can pose a real health threat. With Centers for Disease Control and Prevention (CDC) maps outlining tick ranges throughout the majority of the United States, it’s important we brush up on our understanding of tick-borne diseases. In this article we’ll take a look at the three that pose the biggest risk to horses: Lyme disease, anaplasmosis, and piroplasmosis.
Lyme Disease
Horse owners living in areas of the country heavily infested with Ixodes scapularis, commonly known as blacklegged ticks (also referred to as deer ticks or bear ticks), know these parasites are more than a nuisance. In these regions contracting Lyme disease from infected ticks is entirely possible for horses and humans alike.
Lyme disease is a very difficult disease to prevent, diagnose, and treat in horses, says Linda Mittel, MSPH, DVM, senior extension associate at Cornell University’s Animal Health Diagnostic Center, in Ithaca, New York. Horses contract Lyme disease when the spirochete (a type of bacterium) Borrelia burgdorferi is transmitted through the bite of an infected tick.
“Signs of Lyme disease might not appear until 6 weeks after exposure”
Diagnosis Here’s why diagnosing Lyme disease is tricky: Not all infected horses exhibit clinical signs, and the signs themselves are often confounding, meaning they can point to any number of other diseases. Even if you know an infected tick bit your horse (You can actually test it!), signs of Lyme disease might not appear for up to six weeks after exposure. In short, nothing is straightforward with this disease. An infected tick might or might not transmit the bacteria to its host, and an infected animal might or might not exhibit signs of disease.
So while Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye). While in private practice in the Northeast, Mittel often saw non-neurologic clinical signs of Lyme disease that included unexplained weight loss, changes in attitude and behavior, uveitis, and hypersensitivity. Some signs might arise from the bacterial infection itself, while others (e.g., uveitis) result from the body’s immune response to it. Lyme neuroborreliosis (NB), a rare condition, occurs when B. burgdorferi infects the horse’s nervous system.
Maps courtesy of Centers for Disease Control and Prevention; Tick photos courtesy of Mat Pound/USDA/Bugwood.org and Wikimedia Commons
Learn the latest on diseases horses can get from ticks and why they continue to frustrate veterinarians and researchers
If the sight of a tick makes your skin crawl—even if it’s not crawling on your skin— you’re not alone. That feeling is founded on more than a natural aversion to arachnids; diseases transmitted by ticks can pose a real health threat. With Centers for Disease Control and Prevention (CDC) maps outlining tick ranges throughout the majority of the United States, it’s important we brush up on our understanding of tick-borne diseases. In this article we’ll take a look at the three that pose the biggest risk to horses: Lyme disease, anaplasmosis, and piroplasmosis.
Lyme Disease
Horse owners living in areas of the country heavily infested with Ixodes scapularis, commonly known as blacklegged ticks (also referred to as deer ticks or bear ticks), know these parasites are more than a nuisance. In these regions contracting Lyme disease from infected ticks is entirely possible for horses and humans alike.
Lyme disease is a very difficult disease to prevent, diagnose, and treat in horses, says Linda Mittel, MSPH, DVM, senior extension associate at Cornell University’s Animal Health Diagnostic Center, in Ithaca, New York. Horses contract Lyme disease when the spirochete (a type of bacterium) Borrelia burgdorferi is transmitted through the bite of an infected tick.
“Signs of Lyme disease might not appear until 6 weeks after exposure”
Diagnosis Here’s why diagnosing Lyme disease is tricky: Not all infected horses exhibit clinical signs, and the signs themselves are often confounding, meaning they can point to any number of other diseases. Even if you know an infected tick bit your horse (You can actually test it!), signs of Lyme disease might not appear for up to six weeks after exposure. In short, nothing is straightforward with this disease. An infected tick might or might not transmit the bacteria to its host, and an infected animal might or might not exhibit signs of disease.
So while Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye). While in private practice in the Northeast, Mittel often saw non-neurologic clinical signs of Lyme disease that included unexplained weight loss, changes in attitude and behavior, uveitis, and hypersensitivity. Some signs might arise from the bacterial infection itself, while others (e.g., uveitis) result from the body’s immune response to it. Lyme neuroborreliosis (NB), a rare condition, occurs when B. burgdorferi infects the horse’s nervous system.
 While Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye).
Photo: iStock
Researchers have made strides in Lyme disease diagnostic testing, but there’s still a long way to go. The newest test for B. burgdorferiantibodies (the immune system produces antibodies to fight antigens, or foreign substances, it detects) in the blood is the equine Lyme Multiplex assay developed by the Animal Health Diagnostic Center. This screening identifies three antigen proteins:
While Lyme disease symptoms are not necessarily cut-and-dried, they can include low-grade fever, muscle tenderness, muscle wasting, weight loss, a stiff or uncoordinated gait, swollen joints, “shifting” lameness in various joints, lethargy, hyperesthesia (sensitivity to sound and touch), and uveitis (simply, inflammation of the vascular layer of the eye).
Photo: iStock
Researchers have made strides in Lyme disease diagnostic testing, but there’s still a long way to go. The newest test for B. burgdorferiantibodies (the immune system produces antibodies to fight antigens, or foreign substances, it detects) in the blood is the equine Lyme Multiplex assay developed by the Animal Health Diagnostic Center. This screening identifies three antigen proteins:
 ABOUT THE AUTHOR
Natalie DeFee Mendik, MA
Freelance journalist Natalie DeFee Mendik is a multiple American Horse Publications editorial and graphics awards winner specializing in equestrian media. She holds an MA in English from Colorado State University and an International Federation of Journalists’ International press card, and is a member of the International Alliance of Equestrian Journalists. With over three decades of horse experience, Natalie’s main equine interests are dressage and vaulting. Having lived and ridden in England, Switzerland, and various parts of the United States, Natalie currently resides in Colorado with her husband and two girls.
ABOUT THE AUTHOR
Natalie DeFee Mendik, MA
Freelance journalist Natalie DeFee Mendik is a multiple American Horse Publications editorial and graphics awards winner specializing in equestrian media. She holds an MA in English from Colorado State University and an International Federation of Journalists’ International press card, and is a member of the International Alliance of Equestrian Journalists. With over three decades of horse experience, Natalie’s main equine interests are dressage and vaulting. Having lived and ridden in England, Switzerland, and various parts of the United States, Natalie currently resides in Colorado with her husband and two girls.
